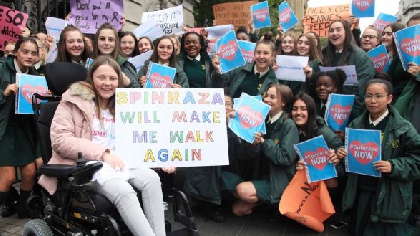
The Minister for Health, Wicklow TD, Simon Harris says he has been assured that there will be no delay in accessing the drug Spinraza, following a lengthy campaign by parents of those affected.
The HSE’s leadership team has approved access to the drug for children with type 1, 2 and 3 Spinal Muscular Atrophy on an exceptional and individualised basis.
Earlier this year, the HSE said the drug would cost about €600,000 in the first year to treat each of the 25 Irish children with the disease and €380,000 a year thereafter,.
Spinraza is now approved in 26 countries around Europe with just Estonia rejecting it.


 Weekend Cold Snap To Provide Brisk Shake Up To Interminable Rainy Monotony
Weekend Cold Snap To Provide Brisk Shake Up To Interminable Rainy Monotony
 Greystones Fishermen Still Struggling Despite Harbour Settlement Warns Senator
Greystones Fishermen Still Struggling Despite Harbour Settlement Warns Senator
 Kilkenny Hurling Legend Helps Wicklow GAA Expand Profile With Fans App
Kilkenny Hurling Legend Helps Wicklow GAA Expand Profile With Fans App
 New Plan Approved For Greystones Delgany Kilcoole.
New Plan Approved For Greystones Delgany Kilcoole.
 Rás Tailteann Returns to Wicklow with Baltinglass Stage Finish
Rás Tailteann Returns to Wicklow with Baltinglass Stage Finish